
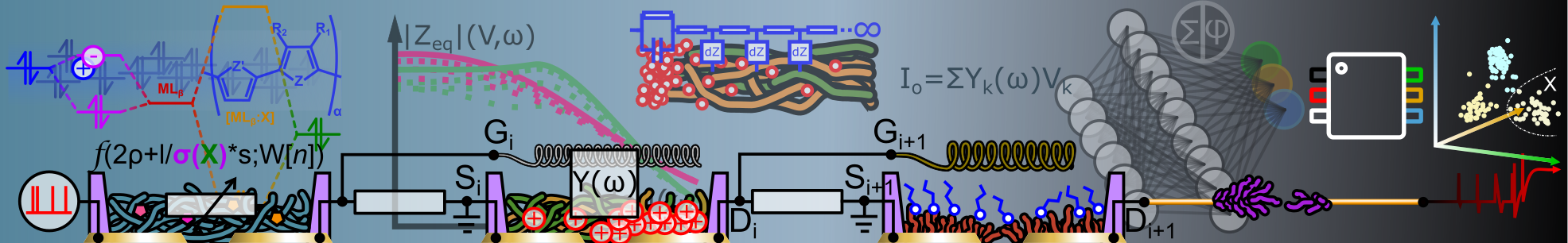




 Abstract: The present invention relates to n-dopants for increasing the electronic conductivity of organic electrical layers, wherein the n-dopant is selected from the group comprising heterocyclic alkali metal salts of the following formula I where X1 - X5 are in dependently selected from the group comprising -CH2-, -CHR-, -CR2-, -C(=O)-, - (C=S) -, - (C=CR2) -, - C(CR)-, =CH-, =CR-, -NH-, -NR-, =N-, -O-, -S-, -Se-, -P(H)-, -P(R)-, -N--, =C--, -CH--, -CR--, -P--, where at least on e Xi provides a heteroatom in the five-membered ring and the ring is formally negatively charged; R is independently selected from the group comprising -H, -D, halogen, -CN, -NO2, -OH, amine, ether, thioether, ester, amide, C1-C50 alkyl, cycloalkyl, acryloyl, vinyl, allyl, aromatic system, fused aromatic system, heteroaromatic system; M = alkali metal or alkaline earth metal and n = 1 or 2.
Abstract: The present invention relates to n-dopants for increasing the electronic conductivity of organic electrical layers, wherein the n-dopant is selected from the group comprising heterocyclic alkali metal salts of the following formula I where X1 - X5 are in dependently selected from the group comprising -CH2-, -CHR-, -CR2-, -C(=O)-, - (C=S) -, - (C=CR2) -, - C(CR)-, =CH-, =CR-, -NH-, -NR-, =N-, -O-, -S-, -Se-, -P(H)-, -P(R)-, -N--, =C--, -CH--, -CR--, -P--, where at least on e Xi provides a heteroatom in the five-membered ring and the ring is formally negatively charged; R is independently selected from the group comprising -H, -D, halogen, -CN, -NO2, -OH, amine, ether, thioether, ester, amide, C1-C50 alkyl, cycloalkyl, acryloyl, vinyl, allyl, aromatic system, fused aromatic system, heteroaromatic system; M = alkali metal or alkaline earth metal and n = 1 or 2.
Kessler F., Pecqueur S., Schmid G.

Abstract: We report on hydrazine-sensing organic electrochemical transistors (OECTs) with a design consisting in concentric annular electrodes. The design engineering of these OECTs was motivated by the great potential of using OECT sensing arrays in fields such a s bioelectronics. In this work, PEDOT:PSS-based OECTs have been studied as aqueous sensors, specifically sensitive to the lethal hydrazine molecule. These amperometric sensors have many relevant features for the development of hydrazine sensors, such as a sensitivity down to 10-5 M of hydrazine in water, an order of magnitude higher selectivity for hydrazine than for 9 other water soluble common analytes, the capability to recover entirely its base signal after water flushing and a very low v oltage operation. The specificity for hydrazine to be sensed by our OECTs is caused by its catalytic oxidation at the gate electrode and enables increasing the output current modulation of the devices. This has permitted the device-geometry study of the whole series of 80 micrometric OECT devices with sub-20-nm PEDOT:PSS layers, channel lengths down to 1 μm and a specific device geometry of coplanar and concentric electrodes. The numerous geometries unravel new aspects of the OECT mechanisms governi ng the electrochemical sensing behaviours of the device, more particularly the effect of the contacts which are inherent at the micro-scale. By lowering the device cross-talking, micrometric gate-integrated radial OECTs shall contribute to the diminishin g of the readout invasiveness and therefore promotes further the development of OECT biosensors.
Pecqueur S., Lenfant S., Guérin D., Alibart F., Vuillaume D.

 Abstract: The invention relates to an n-dopant for doping organic electron transport materials, wherein the n-dopant comprises at least one proazaphosphatrane compound having a three times N-substituted phosphorous atom according to formula 1, wherein R1 - R3 are selected independently from each other from the group R comprising H, D, C1-C60 saturated or unsaturated alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, C1-C60 aryl, alkylaryl, heteroaryl, ether, ester and PR'3, wherein the group R' comprises substitutes of the group R without PR'3, wherein R1 - R3 can be bridged independently from each other; X1 -X3 are selected independently from each other from the group comprising a compound and substituted or non-substi tuted C1-C10 alkyl, cycloalkyl, aryl and alkylaryl.
Abstract: The invention relates to an n-dopant for doping organic electron transport materials, wherein the n-dopant comprises at least one proazaphosphatrane compound having a three times N-substituted phosphorous atom according to formula 1, wherein R1 - R3 are selected independently from each other from the group R comprising H, D, C1-C60 saturated or unsaturated alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, C1-C60 aryl, alkylaryl, heteroaryl, ether, ester and PR'3, wherein the group R' comprises substitutes of the group R without PR'3, wherein R1 - R3 can be bridged independently from each other; X1 -X3 are selected independently from each other from the group comprising a compound and substituted or non-substi tuted C1-C10 alkyl, cycloalkyl, aryl and alkylaryl.
Kessler F., Pecqueur S., Schmid G.

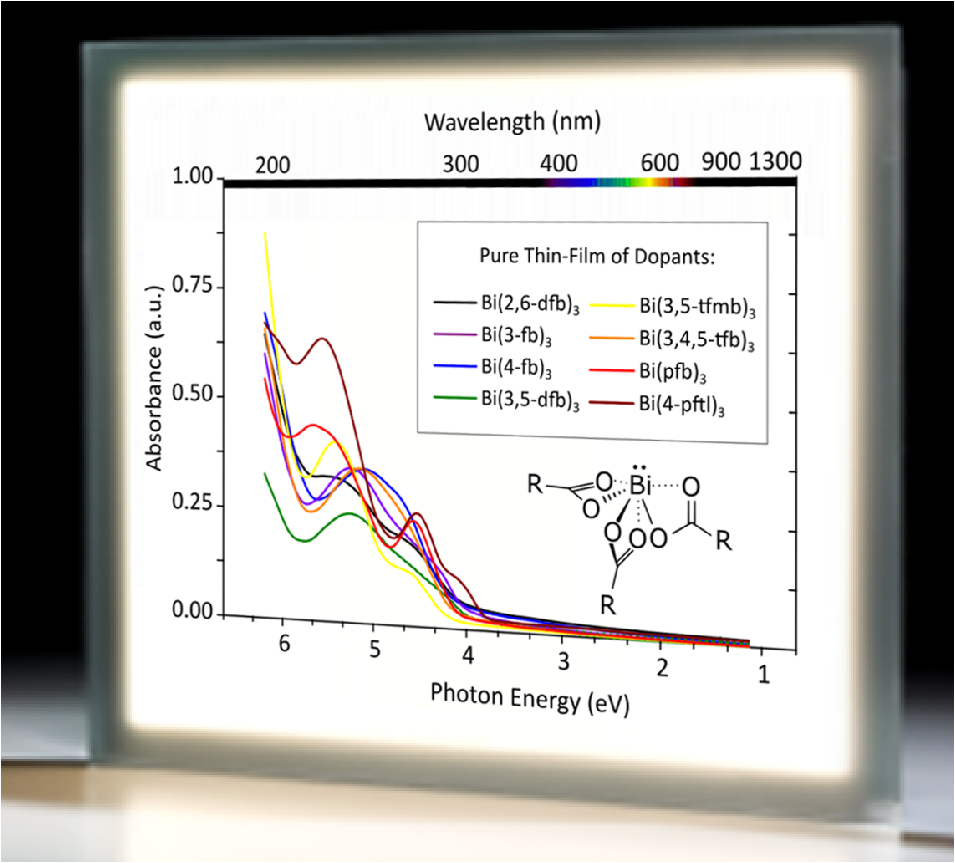 Abstract: Ten new efficient p-dopants for conductivity doping of organic semiconductors for OLEDs are identified. The key advantage of the electrophilic tris(carboxylato) bismuth(III) compounds is the unique low absorption of the resulting doped layers which promo tes the efficiency of OLED devices. The combination of these features with their low fabrication cost, volatility, and stability, make these materials very attractive as dopants in organic electronics.
Abstract: Ten new efficient p-dopants for conductivity doping of organic semiconductors for OLEDs are identified. The key advantage of the electrophilic tris(carboxylato) bismuth(III) compounds is the unique low absorption of the resulting doped layers which promo tes the efficiency of OLED devices. The combination of these features with their low fabrication cost, volatility, and stability, make these materials very attractive as dopants in organic electronics.
Pecqueur S., Maltenberger A., Petrukhina M. A., Halik M., Jaeger A., Pentlehner D., Schmid G.*

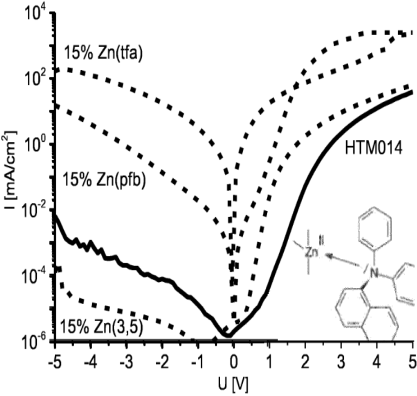 Abstract: The invention relates to an organic electronic component having a matrix containing a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3, and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen-alkyl, ar yl, arylene, halogen-aryl, heteroaryl, heteroarylene, heterocyclic-alkylene, heterocycloalkyl, halogen-heteroaryl, alkenyl, halogen-alkenyl, alkynyl, halogen-alkynyl, ketoaryl, halogen-ketoaryl, ketoheteroaryl, ketoalkyl, halogen-ketoalkyl, ketoalkenyl, halogen-ketoalkenyl, halogen-alkyl-aryl, halogen-alkyl-heteroaryl, wherein, for suitable groups, one or a number of non-adjacent CH2-groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR °°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- o r heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 -groups are understood to be CH2 -groups in the sense of CH2 -H).
Abstract: The invention relates to an organic electronic component having a matrix containing a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3, and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen-alkyl, ar yl, arylene, halogen-aryl, heteroaryl, heteroarylene, heterocyclic-alkylene, heterocycloalkyl, halogen-heteroaryl, alkenyl, halogen-alkenyl, alkynyl, halogen-alkynyl, ketoaryl, halogen-ketoaryl, ketoheteroaryl, ketoalkyl, halogen-ketoalkyl, ketoalkenyl, halogen-ketoalkenyl, halogen-alkyl-aryl, halogen-alkyl-heteroaryl, wherein, for suitable groups, one or a number of non-adjacent CH2-groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR °°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- o r heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 -groups are understood to be CH2 -groups in the sense of CH2 -H).
Kessler F., Maltenberger A., Pecqueur S., Regensburger S., Schmid G.

 Abstract: The invention relates to a method for producing an organic electronic component, wherein the component comprises at least one organic electronic layer having a matrix, wherein the matrix contains a metal complex as a dopant, which metal complex comprises at least one metal atom M and at least one ligand L bonded to the metal atom M, wherein the ligands L have the following structure independently of each other: wherein E1 and E2 can be oxygen, sulfur, selenium, NH, or NR' independently of each other, wh erein R' is selected from the group containing alkyl or aryl and can be bonded to the substituted benzene ring of the ligand L; and the substituents R1 are selected independently of each other from the group comprising branched or unbranched, fluorinated aliphatic hydrocarbons having 1 to 10 C atoms, wherein n = 1 to 5; and the substituents R2 are selected independently of each other from the group comprising -CN, branched or unbranched aliphatic hydrocarbons having 1 to 10 C atoms, aryl, and heteroaryl , wherein m = 0 to at most 5 - n; wherein the deposition of the dopant of the at least one organic electronic layer occurs by means of gas-phase deposition by use of a source, wherein the source is designed in such a way that the dopant experiences impac ts with at least one wall of the source.
Abstract: The invention relates to a method for producing an organic electronic component, wherein the component comprises at least one organic electronic layer having a matrix, wherein the matrix contains a metal complex as a dopant, which metal complex comprises at least one metal atom M and at least one ligand L bonded to the metal atom M, wherein the ligands L have the following structure independently of each other: wherein E1 and E2 can be oxygen, sulfur, selenium, NH, or NR' independently of each other, wh erein R' is selected from the group containing alkyl or aryl and can be bonded to the substituted benzene ring of the ligand L; and the substituents R1 are selected independently of each other from the group comprising branched or unbranched, fluorinated aliphatic hydrocarbons having 1 to 10 C atoms, wherein n = 1 to 5; and the substituents R2 are selected independently of each other from the group comprising -CN, branched or unbranched aliphatic hydrocarbons having 1 to 10 C atoms, aryl, and heteroaryl , wherein m = 0 to at most 5 - n; wherein the deposition of the dopant of the at least one organic electronic layer occurs by means of gas-phase deposition by use of a source, wherein the source is designed in such a way that the dopant experiences impac ts with at least one wall of the source.
Maltenberger A., Pecqueur S., Regensburger S., Schmid G.
© 2019-2025 Sébastien Pecqueur