




 Abstract: The invention relates to an organic electronic component (100) comprising at least one charge generation layer (5) which has an organically p-doped region (5a) that contains a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3; and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen alkyl, at least partially halogenated long-chain alkyl, halogen cycloalkyl, aryl, arylene, halogen aryl, heteroaryl, heteroarylene, heterocyclic alkylene, heterocycloalkyl, halogen heteroaryl, alkenyl, halogen alkenyl, alkynyl, halogen alkynyl, ketoaryl, halogen ketoaryl, ketoheteroaryl, ketoalkyl, halogen ketoalkyl, ketoalkenyl, halogen ketoalkenyl, halogen alkyl aryl, and halogen alkyl heteroaryl, wherein, for suitable gr oups, one or a number of non-adjacent CH2 groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR°°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- or heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 groups are understood to be CH2 groups in the sense of CH2-H). The invention further relates to the use of a zinc complex as a p-dopant in charge generation layers.
Abstract: The invention relates to an organic electronic component (100) comprising at least one charge generation layer (5) which has an organically p-doped region (5a) that contains a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3; and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen alkyl, at least partially halogenated long-chain alkyl, halogen cycloalkyl, aryl, arylene, halogen aryl, heteroaryl, heteroarylene, heterocyclic alkylene, heterocycloalkyl, halogen heteroaryl, alkenyl, halogen alkenyl, alkynyl, halogen alkynyl, ketoaryl, halogen ketoaryl, ketoheteroaryl, ketoalkyl, halogen ketoalkyl, ketoalkenyl, halogen ketoalkenyl, halogen alkyl aryl, and halogen alkyl heteroaryl, wherein, for suitable gr oups, one or a number of non-adjacent CH2 groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR°°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- or heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 groups are understood to be CH2 groups in the sense of CH2-H). The invention further relates to the use of a zinc complex as a p-dopant in charge generation layers.
Kessler F., Maltenberger A., Pecqueur S., Pentlehner D., Regensburger S., Schmid G.

 Abstract: The present invention relates to n-dopants for increasing the electronic conductivity of organic electrical layers, wherein the n-dopant is selected from the group comprising heterocyclic alkali metal salts of the following formula I where X1 - X5 are in dependently selected from the group comprising -CH2-, -CHR-, -CR2-, -C(=O)-, - (C=S) -, - (C=CR2) -, - C(CR)-, =CH-, =CR-, -NH-, -NR-, =N-, -O-, -S-, -Se-, -P(H)-, -P(R)-, -N--, =C--, -CH--, -CR--, -P--, where at least on e Xi provides a heteroatom in the five-membered ring and the ring is formally negatively charged; R is independently selected from the group comprising -H, -D, halogen, -CN, -NO2, -OH, amine, ether, thioether, ester, amide, C1-C50 alkyl, cycloalkyl, acryloyl, vinyl, allyl, aromatic system, fused aromatic system, heteroaromatic system; M = alkali metal or alkaline earth metal and n = 1 or 2.
Abstract: The present invention relates to n-dopants for increasing the electronic conductivity of organic electrical layers, wherein the n-dopant is selected from the group comprising heterocyclic alkali metal salts of the following formula I where X1 - X5 are in dependently selected from the group comprising -CH2-, -CHR-, -CR2-, -C(=O)-, - (C=S) -, - (C=CR2) -, - C(CR)-, =CH-, =CR-, -NH-, -NR-, =N-, -O-, -S-, -Se-, -P(H)-, -P(R)-, -N--, =C--, -CH--, -CR--, -P--, where at least on e Xi provides a heteroatom in the five-membered ring and the ring is formally negatively charged; R is independently selected from the group comprising -H, -D, halogen, -CN, -NO2, -OH, amine, ether, thioether, ester, amide, C1-C50 alkyl, cycloalkyl, acryloyl, vinyl, allyl, aromatic system, fused aromatic system, heteroaromatic system; M = alkali metal or alkaline earth metal and n = 1 or 2.
Kessler F., Pecqueur S., Schmid G.

 Abstract: The invention relates to an n-dopant for doping organic electron transport materials, wherein the n-dopant comprises at least one proazaphosphatrane compound having a three times N-substituted phosphorous atom according to formula 1, wherein R1 - R3 are selected independently from each other from the group R comprising H, D, C1-C60 saturated or unsaturated alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, C1-C60 aryl, alkylaryl, heteroaryl, ether, ester and PR'3, wherein the group R' comprises substitutes of the group R without PR'3, wherein R1 - R3 can be bridged independently from each other; X1 -X3 are selected independently from each other from the group comprising a compound and substituted or non-substi tuted C1-C10 alkyl, cycloalkyl, aryl and alkylaryl.
Abstract: The invention relates to an n-dopant for doping organic electron transport materials, wherein the n-dopant comprises at least one proazaphosphatrane compound having a three times N-substituted phosphorous atom according to formula 1, wherein R1 - R3 are selected independently from each other from the group R comprising H, D, C1-C60 saturated or unsaturated alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, C1-C60 aryl, alkylaryl, heteroaryl, ether, ester and PR'3, wherein the group R' comprises substitutes of the group R without PR'3, wherein R1 - R3 can be bridged independently from each other; X1 -X3 are selected independently from each other from the group comprising a compound and substituted or non-substi tuted C1-C10 alkyl, cycloalkyl, aryl and alkylaryl.
Kessler F., Pecqueur S., Schmid G.

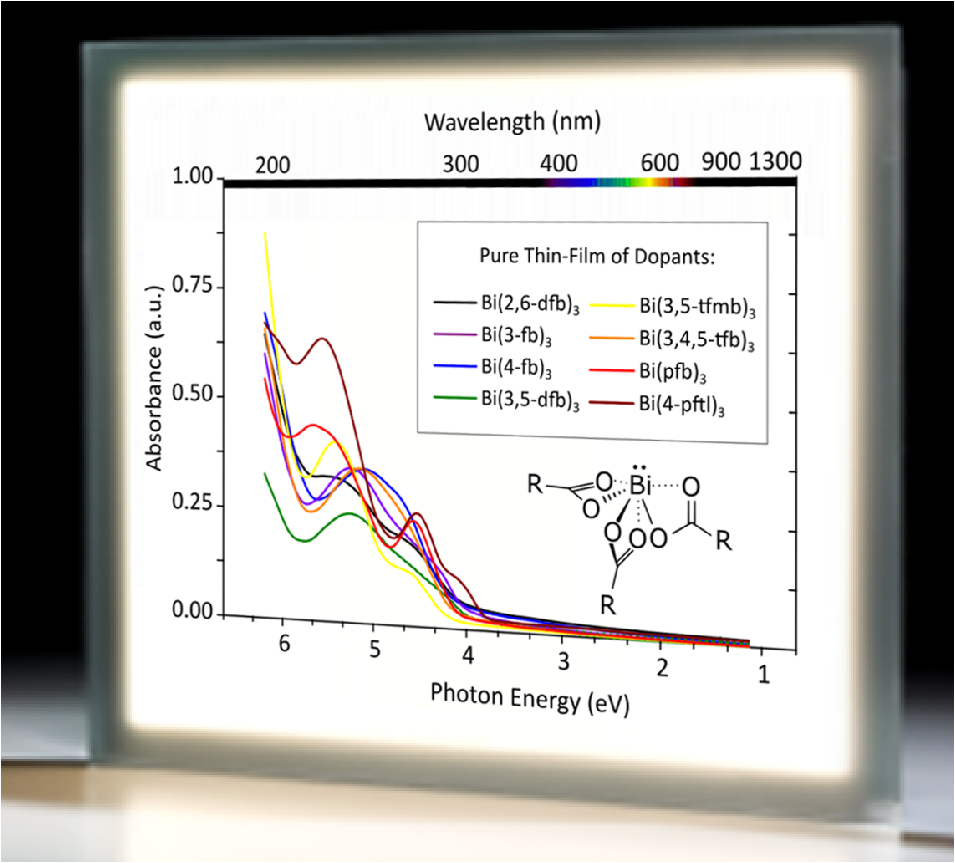 Abstract: Ten new efficient p-dopants for conductivity doping of organic semiconductors for OLEDs are identified. The key advantage of the electrophilic tris(carboxylato) bismuth(III) compounds is the unique low absorption of the resulting doped layers which promo tes the efficiency of OLED devices. The combination of these features with their low fabrication cost, volatility, and stability, make these materials very attractive as dopants in organic electronics.
Abstract: Ten new efficient p-dopants for conductivity doping of organic semiconductors for OLEDs are identified. The key advantage of the electrophilic tris(carboxylato) bismuth(III) compounds is the unique low absorption of the resulting doped layers which promo tes the efficiency of OLED devices. The combination of these features with their low fabrication cost, volatility, and stability, make these materials very attractive as dopants in organic electronics.
Pecqueur S., Maltenberger A., Petrukhina M. A., Halik M., Jaeger A., Pentlehner D., Schmid G.*

 Abstract: The invention relates to an organic electronic component having a matrix containing a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3, and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen-alkyl, ar yl, arylene, halogen-aryl, heteroaryl, heteroarylene, heterocyclic-alkylene, heterocycloalkyl, halogen-heteroaryl, alkenyl, halogen-alkenyl, alkynyl, halogen-alkynyl, ketoaryl, halogen-ketoaryl, ketoheteroaryl, ketoalkyl, halogen-ketoalkyl, ketoalkenyl, halogen-ketoalkenyl, halogen-alkyl-aryl, halogen-alkyl-heteroaryl, wherein, for suitable groups, one or a number of non-adjacent CH2-groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR °°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- o r heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 -groups are understood to be CH2 -groups in the sense of CH2 -H).
Abstract: The invention relates to an organic electronic component having a matrix containing a zinc complex as a p-dopant, said zinc complex in turn containing at least one ligand L of the following structure: formula (I) wherein R1 and R2 can be oxygen, sulphur, selenium, NH or NR4 independently from one another, wherein R4 is selected from the group containing alkyl or aryl and which can be bonded to R3, and wherein R3 is selected from the group containing alkyl, long-chain alkyl, cycloalkyl, halogen-alkyl, ar yl, arylene, halogen-aryl, heteroaryl, heteroarylene, heterocyclic-alkylene, heterocycloalkyl, halogen-heteroaryl, alkenyl, halogen-alkenyl, alkynyl, halogen-alkynyl, ketoaryl, halogen-ketoaryl, ketoheteroaryl, ketoalkyl, halogen-ketoalkyl, ketoalkenyl, halogen-ketoalkenyl, halogen-alkyl-aryl, halogen-alkyl-heteroaryl, wherein, for suitable groups, one or a number of non-adjacent CH2-groups can be replaced by -O-, -S-, -NH-, -NR°°°-, -SiR°R°°-, -CO-, -COO-, -COR°OR °°-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -O-CS-, -CS-O-, -CY1=CY2 or -C≡C- independently from one another, and in such a way that O and/or S atoms are not directly bonded to one another, and are replaced optionally with aryl- o r heteroaryl preferably containing between 1 and 30 C atoms (terminal CH3 -groups are understood to be CH2 -groups in the sense of CH2 -H).
Kessler F., Maltenberger A., Pecqueur S., Regensburger S., Schmid G.

 Abstract: The invention relates to a method for producing an organic electronic component, wherein the component comprises at least one organic electronic layer having a matrix, wherein the matrix contains a metal complex as a dopant, which metal complex comprises at least one metal atom M and at least one ligand L bonded to the metal atom M, wherein the ligands L have the following structure independently of each other: wherein E1 and E2 can be oxygen, sulfur, selenium, NH, or NR' independently of each other, wh erein R' is selected from the group containing alkyl or aryl and can be bonded to the substituted benzene ring of the ligand L; and the substituents R1 are selected independently of each other from the group comprising branched or unbranched, fluorinated aliphatic hydrocarbons having 1 to 10 C atoms, wherein n = 1 to 5; and the substituents R2 are selected independently of each other from the group comprising -CN, branched or unbranched aliphatic hydrocarbons having 1 to 10 C atoms, aryl, and heteroaryl , wherein m = 0 to at most 5 - n; wherein the deposition of the dopant of the at least one organic electronic layer occurs by means of gas-phase deposition by use of a source, wherein the source is designed in such a way that the dopant experiences impac ts with at least one wall of the source.
Abstract: The invention relates to a method for producing an organic electronic component, wherein the component comprises at least one organic electronic layer having a matrix, wherein the matrix contains a metal complex as a dopant, which metal complex comprises at least one metal atom M and at least one ligand L bonded to the metal atom M, wherein the ligands L have the following structure independently of each other: wherein E1 and E2 can be oxygen, sulfur, selenium, NH, or NR' independently of each other, wh erein R' is selected from the group containing alkyl or aryl and can be bonded to the substituted benzene ring of the ligand L; and the substituents R1 are selected independently of each other from the group comprising branched or unbranched, fluorinated aliphatic hydrocarbons having 1 to 10 C atoms, wherein n = 1 to 5; and the substituents R2 are selected independently of each other from the group comprising -CN, branched or unbranched aliphatic hydrocarbons having 1 to 10 C atoms, aryl, and heteroaryl , wherein m = 0 to at most 5 - n; wherein the deposition of the dopant of the at least one organic electronic layer occurs by means of gas-phase deposition by use of a source, wherein the source is designed in such a way that the dopant experiences impac ts with at least one wall of the source.
Maltenberger A., Pecqueur S., Regensburger S., Schmid G.

 Abstract: The invention relates to a method for producing hole-transporting electrical layers, wherein a functionalized organic matrix compound is reacted with at least one cross-linking reagent on a substrate, higher-molecular-weight compounds thus being formed, wherein the functionalized organic matrix compound corresponds to the following formula 1, wherein L is a bond or is selected from the group comprising substituted or unsubstituted, saturated or unsaturated C1-C50 alkyl, aryl, polye thylene glycol, polyethylene diamine, polyester, polyurethane, or polyvinylidene phenyl chains or mixtures thereof; E1 and E2 can be oxygen, sulfur, selenium, NH, or NE3 independently of each other, wherein E3 is selected from the group compri sing substituted or unsubstituted alkyl or aryl, wherein E3 can be bonded to R; R is selected from the group comprising H, D, C1-C10 alkyl or aryl silyl ester, fluorinated or unfluorinated branched or unbranched C1-C 10 alkyl, aryl, or heteroaryl, and RHTL is the backbone of an organic hole transporter, and the cross-linking reagent comprises at least one metal atom from groups 13-15 and at least one organic ligand.
Abstract: The invention relates to a method for producing hole-transporting electrical layers, wherein a functionalized organic matrix compound is reacted with at least one cross-linking reagent on a substrate, higher-molecular-weight compounds thus being formed, wherein the functionalized organic matrix compound corresponds to the following formula 1, wherein L is a bond or is selected from the group comprising substituted or unsubstituted, saturated or unsaturated C1-C50 alkyl, aryl, polye thylene glycol, polyethylene diamine, polyester, polyurethane, or polyvinylidene phenyl chains or mixtures thereof; E1 and E2 can be oxygen, sulfur, selenium, NH, or NE3 independently of each other, wherein E3 is selected from the group compri sing substituted or unsubstituted alkyl or aryl, wherein E3 can be bonded to R; R is selected from the group comprising H, D, C1-C10 alkyl or aryl silyl ester, fluorinated or unfluorinated branched or unbranched C1-C 10 alkyl, aryl, or heteroaryl, and RHTL is the backbone of an organic hole transporter, and the cross-linking reagent comprises at least one metal atom from groups 13-15 and at least one organic ligand.
Maltenberger A., Pecqueur S., Schmid G.

Abstract:
Schmid G., Pecqueur S., Halik M.

 Abstract: The invention relates to a bi- or polynuclear metal complex of a metal of groups Vb/VIb/VIIb, or rather groups 5-7, having at least one ligand of the structure (a), wherein R1 and R2, independently of each other, can be oxygen, sulfur, selenium, NH, or N R4, wherein R4 is selected from the group containing alkyl or aryl and can be bonded to R3; and R3 is selected from the group containing alkyl, long-chain alkyl, alkoxy, long-chain alkoxy, cycloalkyl, haloalkyl, aryl, arylenes, haloaryl, heteroaryl, hete roarylenes, heterocycloalkylenes, heterocycloalkyl, haloheteroaryl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, ketoaryl, haloketoaryl, ketoheteroaryl, ketoalkyl, haloketoalkyl, ketoalkenyl, haloketoalkenyl, wherein for suitable residues, one or more non -adjacent CH2 groups can be replaced, independently of each other, with -O-, -S-, -NH-, -NR°-, -SiR°R°°-, -CO-, -COO-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -CY1=CY2, or -C≡C- in such a way that O and/or S atom s are not bonded directly to each other, likewise optionally are replaced with aryl or heteroaryl preferably containing 1 to 30 C atoms, as a p-type doping agent for matrix materials of electronic components.
Abstract: The invention relates to a bi- or polynuclear metal complex of a metal of groups Vb/VIb/VIIb, or rather groups 5-7, having at least one ligand of the structure (a), wherein R1 and R2, independently of each other, can be oxygen, sulfur, selenium, NH, or N R4, wherein R4 is selected from the group containing alkyl or aryl and can be bonded to R3; and R3 is selected from the group containing alkyl, long-chain alkyl, alkoxy, long-chain alkoxy, cycloalkyl, haloalkyl, aryl, arylenes, haloaryl, heteroaryl, hete roarylenes, heterocycloalkylenes, heterocycloalkyl, haloheteroaryl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, ketoaryl, haloketoaryl, ketoheteroaryl, ketoalkyl, haloketoalkyl, ketoalkenyl, haloketoalkenyl, wherein for suitable residues, one or more non -adjacent CH2 groups can be replaced, independently of each other, with -O-, -S-, -NH-, -NR°-, -SiR°R°°-, -CO-, -COO-, -OCO-, -OCO-O-, -SO2-, -S-CO-, -CO-S-, -CY1=CY2, or -C≡C- in such a way that O and/or S atom s are not bonded directly to each other, likewise optionally are replaced with aryl or heteroaryl preferably containing 1 to 30 C atoms, as a p-type doping agent for matrix materials of electronic components.
Maltenberger A., Pecqueur S., Schmid G., Wemken J. H.

 Abstract: The invention relates to an organic electron transport layer n-dopant, the use of said n-dopant to construct organic electronic components, transistors, organic light-emitting diodes, light-emitting electrochemical cells, organic solar cells, photodiodes , and electronic components containing said n-dopant.
Abstract: The invention relates to an organic electron transport layer n-dopant, the use of said n-dopant to construct organic electronic components, transistors, organic light-emitting diodes, light-emitting electrochemical cells, organic solar cells, photodiodes , and electronic components containing said n-dopant.
Kanitz A., Pecqueur S., Schmid G., Wemken J. H.

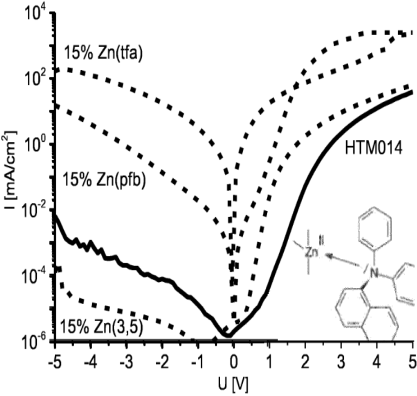
Abstract: Organic electronic is up to now the most promising technology in order to realize opto electronic devices suitable on flexible substrates, which can open new markets on plastic-based products. Nevertheless, to compete classic technologies on already exis ting markets, organic electronic needs to improve several of its electrical performances among others. Doping organic semiconductors is one strategy to optimize electrical conductivity on organic materials but is still very limiting compared to inorganic , and understanding the complex mechanism between dopant and organic semiconductor is a prerequisite for their optimization. Even if the experience shows classic dopants to be redox-active chemicals (Cs, Li, O2), the redox activity of some che micals is no prerequisite for doping. Despite its strong reducing property, Cr2(tfa)4 has been demonstrated to be a p-dopant for its Lewis acidity. Cr2(tfa)4 presents an air-sensitivity due to the redox-activit y of the core, which implies that the conception of Lewis acids and bases, stable under oxidizing or reducing conditions,can result in potential air-stable materials which would dope organic semiconductors by the formation of hybrid charge-transfer compl exes.
Pecqueur S., Halik M., Schmid G.
© 2019-2025 Sébastien Pecqueur