
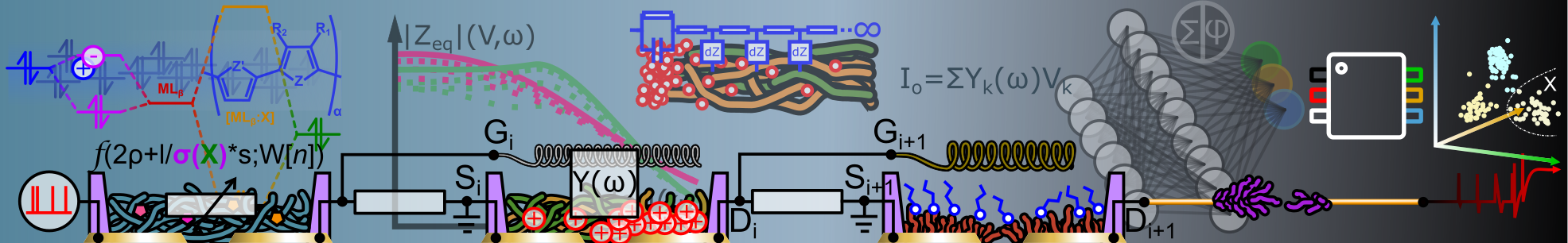




Abstract: The development of electronic devices for neurosensing is leading to fundamental discoveries in communication setups for interfacing and computing the brain's electrical activity that is still a demanding task in the 21st century. One of the greatest cha llenges for efficient neurosensing is to ensure that detection/transduction between biochemically rich systems and tools is fully mastered to reliably gather relevant information. In extracellular devices such as microelectrode arrays (MEAs), the discord ance lies at the interface between ions and the electrodes. Engineering chemically/morphologically the electrode's materials by decreasing its impedance, improving its affinity with neurons, and boosting its biocompatibility ensures better cell/electrode interface conditions to find the right materials that detect ionic signals from neurons and transduce them into electronic signals with the lowest information loss. Hence, the use of conducting polymers (PEDOT) has emerged for optimizing the performance of microelectrodes in neurosensing due to its mixed ionic electronic conduction, biocompatibility and low impedance. In parallel to the development of passive microelectrode, organic electrochemical transistors (OECTs) have received lots of attention in the biosensing field since they exhibit high coupling with cells and signal amplification. Notably, the transconductance represents an important parameter that depends on geometrical and material parameters that rules largely OECTs performances in biose nsing. In this direction, we explore the use of EDOT electropolymerization to tune post-fabrication material and geometrical parameters of passive microelectrodes for optimizing the cell/electrode interface by decreasing its impedance and improving its a ffinity with neurons (increasing the resistance "Rseal" that represents the cell/electrode cleft). For electropolymerized PEDOT MEAs, we demonstrate long term and stable extracellular recording of primary cortical neurons with a record signal-to-noise ra tio (SNR) up to 37 dB (with ultra-low noise down to 2.1 μV RMS). Secondly, for active sensing with OECTs, this strategy exploits the concept of adaptive sensing where both transconductance and impedance are tuned simultaneously or independently. This approach shows an improvement of OECTs transconductance by 150-percent, volumetric capacitance by 300-percent, and a reduction in array's variability by 60-percent in comparison with standard spin-coated OECTs. The cytotoxicity of the electropolymerized EDOT was assessed for primary neural cells culture and no detrimental effect of electropolymerized EDOT on cell viability was observed. To extract the impedance and transconductance values for both MEAs and OECTs, we combine DC electrical measurements w ith electrochemical impedance spectroscopy (EIS). To show the cell/electrode morphology and neurite outgrowth to electropolymerized microelectrodes, Scanning Electron Microscopy (SEM) was performed. To correlate the morphological changes of the material with the enhancement of its electrical and electrochemical performances, Atomic Force Microscopy (AFM) in liquid and Raman Spectroscopy were achieved. Finally, in-vitro extracellular recorded signals from entorhinal cortex cultured slices and primary cor tical neurons using both MEAs and OECTs are presented. The key novelty of this technique is to propose a post-fabrication material engineering technique that can be used to optimize both passive (MEAs) and active (OECTs) devices for extracellular recordi ng and promote new exploratory sensing strategies to ensure high quality neurosensing alternatives.
Ghazal M., Scholaert C., Daher Mansour M., Janel S., Barois N., Halliez S., Dargent T., Coffinier Y., Pecqueur S., Alibart F.



 Abstract: Organic electrochemical transistors are considered today as a key technology to interact with a biological medium through their intrinsic ionic-electronic coupling. In this paper, the authors show how this coupling can be finely tuned (in operando) post- microfabrication via the electropolymerization technique. This strategy exploits the concept of adaptive sensing where both transconductance and impedance are tunable and can be modified on-demand to match different sensing requirements. Material investi gation through Raman spectroscopy, atomic force microscopy, and scanning electron microscopy reveals that electropolymerization can lead to a fine control of poly(3,4-ethylenedioxythiophene) (PEDOT) microdomains organization, which directly affects the i ono-electronic properties of organic electrochemical transistors (OECTs). They further highlight how volumetric capacitance and effective mobility of PEDOT:polystyrene sulfonate influence distinctively the transconductance and impedance of OECTs. This ap proach shows to improve the transconductance by 150% while reducing their variability by 60\% in comparison with standard spin-coated OECTs. Finally, they show how the technique can influence voltage spike rate hardware classification with direct interes t in bio-signals sorting applications.
Abstract: Organic electrochemical transistors are considered today as a key technology to interact with a biological medium through their intrinsic ionic-electronic coupling. In this paper, the authors show how this coupling can be finely tuned (in operando) post- microfabrication via the electropolymerization technique. This strategy exploits the concept of adaptive sensing where both transconductance and impedance are tunable and can be modified on-demand to match different sensing requirements. Material investi gation through Raman spectroscopy, atomic force microscopy, and scanning electron microscopy reveals that electropolymerization can lead to a fine control of poly(3,4-ethylenedioxythiophene) (PEDOT) microdomains organization, which directly affects the i ono-electronic properties of organic electrochemical transistors (OECTs). They further highlight how volumetric capacitance and effective mobility of PEDOT:polystyrene sulfonate influence distinctively the transconductance and impedance of OECTs. This ap proach shows to improve the transconductance by 150% while reducing their variability by 60\% in comparison with standard spin-coated OECTs. Finally, they show how the technique can influence voltage spike rate hardware classification with direct interes t in bio-signals sorting applications.
Ghazal M., Daher Mansour M., Scholaert C., Dargent T., Coffinier Y., Pecqueur S.*, Alibart F.*

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract:
Ghazal M., Daher Mansour M., Halliez S., Coffinier Y., Dargent T., Pecqueur S., Alibart F.

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract: Microelectrode arrays (MEAs) are widely used tools for investigating neural activity. To ensure the best sensitivity of the electronic devices to ionic signals and the lowest information loss, their electrochemical interface must be optimized by lowering their surface impedance, with materials that ensure the highest compatibility with the cells at the same time. Here, we show that by the electropolymerization of thiophene-derivatives, functionalized for higher cell biocompatibility and higher electroch emical performances, one can lower the microelectrodes' surface impedance by the control of the polymer morphology. The microelectrode structuring with bottom-up grown conducting polymers was monitored in-situ by voltage-ramped impedance spectroscopy upo n electropolymerization to track its circuit-elements modification. Iterative impedance modeling over the growth confirmed the material's electrochemical dynamic to be controlled by the gradual modifications of specific discrete circuit elements at diffe rent frequency ranges, thanks to the surface electrodes microstructuring. More particularly, we systematically evidenced a monotonic change of the electrode charging from ideal capacitor to constant phase element dominated modes, due to the bulk charging of the conducting polymer. The evolution of the materials morphology screened by atomic force microscopy and electron microscopy has been confronted to the modification of the materials circuit element, and confirmed distinctive charging modes for the e lectrodes that are governed by their different texturing. In addition to the surface morphology, chemical tuning of the electrodeposited polymer has been performed and showed that a fine tuning of the polymer's glycolation promotes the decrease of the el ectrodes' electrochemical impedance down to -15% compared to the unglycolated polymers thanks to a right balance between ionic permeability and electronic performances. Overall, lower impedance values than commercial MEAs have been systematically reached with performances comparable to spin-coated polymer electrodes', and with low performance dispersion over the whole population of electrodes in the MEAs. With the presented preliminary biocompatibility and stability tests, this study aims is to demonstr ate that unusual microfabrication techniques derived from electrochemistry can provide unique features at the material level to match properties of future emerging bioelectronics technologies to the strong requirements of sensing involving biological mat erials with rich material chemistry and morphology. This work paves the way to new approaches for neuromorphic engineering, such as structural plasticity and neural network topology exploration.
Susloparova A., Ghazal M., Guérin D., Halliez S., Coffinier Y., Dargent T., Alibart F., Pecqueur S.

Abstract: The recent progress in the extracellular microelectrode arrays (MEAs) have greatly improved our ability to probe cellular electrophysiological activities. Nevertheless, passive MEAs are subject to small signal-to-noise ratio and small potential detection . Recently, organic electrochemical transistors (OECTs) have been identified as a promising device architecture to improve extracellular potentials recording in electroactive cells culture both in-vitro and in-vivo. In addition to unique properties of in terest for electrophysiology such as biocompatibility, transparency and flexibility, OECTs operating principle is based on the transduction of ionic currents in the biological medium into electronic currents in the organic semiconductor (e.g. PEDOT:PSS) via electrochemical coupling. The transconductance represents an important figure of merit of OECTs and depends on geometrical and material parameters that rules largely OECTs performances for sensing electrophysiological signals. However, as an organic electronic technology, larger device-property distributions are often encountered with respect to the one of metal- or inorganic-based technologies, inherent to the very nature of the soft organic materials involved in the OECTs transduction process. Her e, we explore the possibility to tune post-fabrication material and geometrical parameters of OECTs with electropolymerization of EDOT. We show that this strategy can be used to simultaneously improve OECT transconductance and its geometrical capacitance . The addressed OECT chips were micro-fabricated on a glass substrates with spin coated PEDOT:PSS. Electropolymerization of EDOT on top of spin-coated PEDOT:PSS was carried on with both fix voltage and ramp voltage techniques. A detail impedance analysis was performed during OECTs functionalization. DC electrical characterizations was used to correlate the transconductance and capacitance tuning due to electropolymerization and to assess device performances improvements. Scanning Electron Microscopy (SE M) was used to correlate morphological changes due to electropolymerization with the enhancement in the transconductance and capacitance of the OECTs. Finally, we performed bio-compatibility assessment between primary neural cells culture and the differe nt possible monomers used for electropolymerization to evaluate the possibility to improve affinity between cultured neurons and electropolymerized materials. The key novelty of this material engineering technique is to propose a promising method for tun able OECTs sensors development. For instance, this back-end-of-line tuning technique can reduce chip variability in terms of performance yield and bring OECTs technology to the next maturity level. Furthermore, such flexibility can enable matching the el ectrochemical impedance of the device to the one of the cells, and in the future promote exploratory sensing missions, merging brain-inspired information processing with neuro-sensing.},
Ghazal M., Susloparova A., Halliez S., Colin M., Buée L., Coffinier Y., Pecqueur S., Dargent T., Alibart F.


Abstract: We report on the comparison between two different driving circuits for addressing micro-fabricated organic electrochemical transistors of different channel resistances and transconductance, aiming for neuromorphic sensing. The Wheatstone bridge configura tion shows interesting results by offering more versatility towards higher resistance materials. However, the Current-Voltage converter observed faster transients. Both circuits show different assets very encouraging for further practical application.
Ghazal M., Dargent T., Pecqueur S., Alibart F.
© 2019-2025 Sébastien Pecqueur