
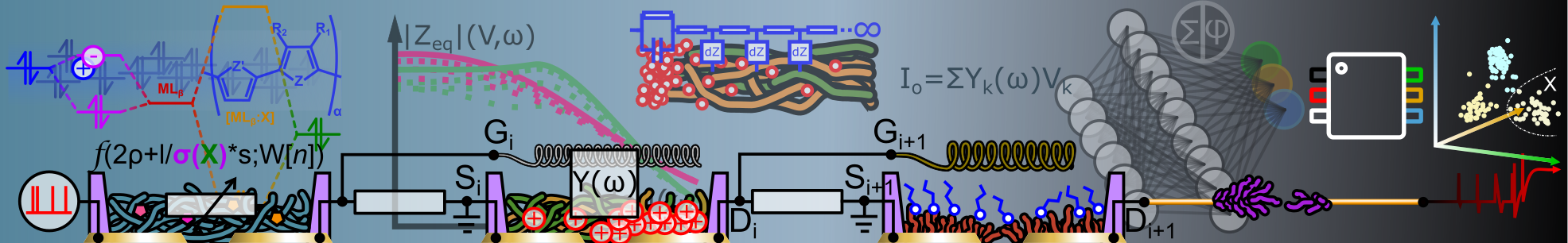





 Abstract: Microelectrode Arrays (MEAs) are popular tools for in vitro extracellular recording. They are often optimized by surface engineering to improve affinity with neurons and guarantee higher recording quality and stability. Recently, PEDOT:PSS has been used to coat microelectrodes due to its good biocompatibility and low impedance, which enhances neural coupling. Herein, we investigate on electro-co-polymerization of EDOT with its triglymated derivative to control valence between monomer units and hydrophil ic functions on a conducting polymer. Molecular packing, cation complexation, dopant stoichiometry are governed by the glycolation degree of the electro-active coating of the microelectrodes. Optimal monomer ratio allows fine-tuning the material hydrophi licity and biocompatibility without compromising the electrochemical impedance of microelectrodes nor their stability while interfaced with a neural cell culture. After incubation, sensing readout on the modified electrodes shows higher performances with respect to unmodified electropolymerized PEDOT, with higher signal-to-noise ratio (SNR) and higher spike counts on the same neural culture. Reported SNR values are superior to that of state-of-theart PEDOT microelectrodes and close to that of state-of-t he-art 3D microelectrodes, with a reduced fabrication complexity. Thanks to this versatile technique and its impact on the surface chemistry of the microelectrode, we show that electro-co-polymerization trades with manycompound properties to easily gathe r them into single macromolecular structures. Applied on sensor arrays, it holds great potential for the customization of neurosensors to adapt to environmental boundaries and to optimize extracted sensing features.
Abstract: Microelectrode Arrays (MEAs) are popular tools for in vitro extracellular recording. They are often optimized by surface engineering to improve affinity with neurons and guarantee higher recording quality and stability. Recently, PEDOT:PSS has been used to coat microelectrodes due to its good biocompatibility and low impedance, which enhances neural coupling. Herein, we investigate on electro-co-polymerization of EDOT with its triglymated derivative to control valence between monomer units and hydrophil ic functions on a conducting polymer. Molecular packing, cation complexation, dopant stoichiometry are governed by the glycolation degree of the electro-active coating of the microelectrodes. Optimal monomer ratio allows fine-tuning the material hydrophi licity and biocompatibility without compromising the electrochemical impedance of microelectrodes nor their stability while interfaced with a neural cell culture. After incubation, sensing readout on the modified electrodes shows higher performances with respect to unmodified electropolymerized PEDOT, with higher signal-to-noise ratio (SNR) and higher spike counts on the same neural culture. Reported SNR values are superior to that of state-of-theart PEDOT microelectrodes and close to that of state-of-t he-art 3D microelectrodes, with a reduced fabrication complexity. Thanks to this versatile technique and its impact on the surface chemistry of the microelectrode, we show that electro-co-polymerization trades with manycompound properties to easily gathe r them into single macromolecular structures. Applied on sensor arrays, it holds great potential for the customization of neurosensors to adapt to environmental boundaries and to optimize extracted sensing features.
Ghazal M., Susloparova A., Lefebvre C., Daher Mansour M., Ghodhbane N., Melot A., Scholaert C., Guérin D., Janel S., Barois N., Colin M., Buée L., Yger P., Halliez S., Coffinier Y.*, Pecqueur S.*, Alibart F.


 (dataset:
(dataset:  )
) Abstract: Although materials and processes are different from biological cells', brain mimicries led to tremendous achievements in parallel information processing via neuromorphic engineering. Inexistent in electronics, we emulate dendritic morphogenesis by electr opolymerization in water, aiming in operando material modification for hardware learning. Systematic study of applied voltage-pulse parameters details on tuning independently morphological aspects of micrometric dendrites': fractal number, branching degr ee, asymmetry, density or length. Growths time-lapse image processing shows spatial features to be dynamically dependent, and expand distinctively before and after conductive bridging with two electro-generated dendrites. Circuit-element analysis and imp edance spectroscopy confirms their morphological control in temporal windows where growth kinetics is finely perturbed by the input frequency and duty cycle. By the emulation of one's most preponderant mechanisms for brain's long-term memory, its impleme ntation in vicinity of sensing arrays, neural probes or biochips shall greatly optimize computational costs and recognition required to classify high-dimensional patterns from complex environments.
Abstract: Although materials and processes are different from biological cells', brain mimicries led to tremendous achievements in parallel information processing via neuromorphic engineering. Inexistent in electronics, we emulate dendritic morphogenesis by electr opolymerization in water, aiming in operando material modification for hardware learning. Systematic study of applied voltage-pulse parameters details on tuning independently morphological aspects of micrometric dendrites': fractal number, branching degr ee, asymmetry, density or length. Growths time-lapse image processing shows spatial features to be dynamically dependent, and expand distinctively before and after conductive bridging with two electro-generated dendrites. Circuit-element analysis and imp edance spectroscopy confirms their morphological control in temporal windows where growth kinetics is finely perturbed by the input frequency and duty cycle. By the emulation of one's most preponderant mechanisms for brain's long-term memory, its impleme ntation in vicinity of sensing arrays, neural probes or biochips shall greatly optimize computational costs and recognition required to classify high-dimensional patterns from complex environments.
Janzakova K., Kumar A., Ghazal M., Susloparova A., Coffinier Y., Alibart F., Pecqueur S.*

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract: Microelectrode arrays (MEAs) are widely used tools for investigating neural activity. To ensure the best sensitivity of the electronic devices to ionic signals and the lowest information loss, their electrochemical interface must be optimized by lowering their surface impedance, with materials that ensure the highest compatibility with the cells at the same time. Here, we show that by the electropolymerization of thiophene-derivatives, functionalized for higher cell biocompatibility and higher electroch emical performances, one can lower the microelectrodes' surface impedance by the control of the polymer morphology. The microelectrode structuring with bottom-up grown conducting polymers was monitored in-situ by voltage-ramped impedance spectroscopy upo n electropolymerization to track its circuit-elements modification. Iterative impedance modeling over the growth confirmed the material's electrochemical dynamic to be controlled by the gradual modifications of specific discrete circuit elements at diffe rent frequency ranges, thanks to the surface electrodes microstructuring. More particularly, we systematically evidenced a monotonic change of the electrode charging from ideal capacitor to constant phase element dominated modes, due to the bulk charging of the conducting polymer. The evolution of the materials morphology screened by atomic force microscopy and electron microscopy has been confronted to the modification of the materials circuit element, and confirmed distinctive charging modes for the e lectrodes that are governed by their different texturing. In addition to the surface morphology, chemical tuning of the electrodeposited polymer has been performed and showed that a fine tuning of the polymer's glycolation promotes the decrease of the el ectrodes' electrochemical impedance down to -15% compared to the unglycolated polymers thanks to a right balance between ionic permeability and electronic performances. Overall, lower impedance values than commercial MEAs have been systematically reached with performances comparable to spin-coated polymer electrodes', and with low performance dispersion over the whole population of electrodes in the MEAs. With the presented preliminary biocompatibility and stability tests, this study aims is to demonstr ate that unusual microfabrication techniques derived from electrochemistry can provide unique features at the material level to match properties of future emerging bioelectronics technologies to the strong requirements of sensing involving biological mat erials with rich material chemistry and morphology. This work paves the way to new approaches for neuromorphic engineering, such as structural plasticity and neural network topology exploration.
Susloparova A., Ghazal M., Guérin D., Halliez S., Coffinier Y., Dargent T., Alibart F., Pecqueur S.

Abstract: The recent progress in the extracellular microelectrode arrays (MEAs) have greatly improved our ability to probe cellular electrophysiological activities. Nevertheless, passive MEAs are subject to small signal-to-noise ratio and small potential detection . Recently, organic electrochemical transistors (OECTs) have been identified as a promising device architecture to improve extracellular potentials recording in electroactive cells culture both in-vitro and in-vivo. In addition to unique properties of in terest for electrophysiology such as biocompatibility, transparency and flexibility, OECTs operating principle is based on the transduction of ionic currents in the biological medium into electronic currents in the organic semiconductor (e.g. PEDOT:PSS) via electrochemical coupling. The transconductance represents an important figure of merit of OECTs and depends on geometrical and material parameters that rules largely OECTs performances for sensing electrophysiological signals. However, as an organic electronic technology, larger device-property distributions are often encountered with respect to the one of metal- or inorganic-based technologies, inherent to the very nature of the soft organic materials involved in the OECTs transduction process. Her e, we explore the possibility to tune post-fabrication material and geometrical parameters of OECTs with electropolymerization of EDOT. We show that this strategy can be used to simultaneously improve OECT transconductance and its geometrical capacitance . The addressed OECT chips were micro-fabricated on a glass substrates with spin coated PEDOT:PSS. Electropolymerization of EDOT on top of spin-coated PEDOT:PSS was carried on with both fix voltage and ramp voltage techniques. A detail impedance analysis was performed during OECTs functionalization. DC electrical characterizations was used to correlate the transconductance and capacitance tuning due to electropolymerization and to assess device performances improvements. Scanning Electron Microscopy (SE M) was used to correlate morphological changes due to electropolymerization with the enhancement in the transconductance and capacitance of the OECTs. Finally, we performed bio-compatibility assessment between primary neural cells culture and the differe nt possible monomers used for electropolymerization to evaluate the possibility to improve affinity between cultured neurons and electropolymerized materials. The key novelty of this material engineering technique is to propose a promising method for tun able OECTs sensors development. For instance, this back-end-of-line tuning technique can reduce chip variability in terms of performance yield and bring OECTs technology to the next maturity level. Furthermore, such flexibility can enable matching the el ectrochemical impedance of the device to the one of the cells, and in the future promote exploratory sensing missions, merging brain-inspired information processing with neuro-sensing.},
Ghazal M., Susloparova A., Halliez S., Colin M., Buée L., Coffinier Y., Pecqueur S., Dargent T., Alibart F.


 Abstract: In this study, we present the microfabrication and characterization of a transparent microelectrode array (MEA) system based on PEDOT:PSS for electrophysiology. The influence of the PEDOT:PSS electrode dimensions on the impedance was investigated and the stability over time under physiological environment was demonstrated. A very good transparency value was obtained by our system displaying one of the best impedance and transmittance values when compared to other transparent MEA. After biocompatibility validation, we successfully recorded spontaneous neuronal activity of primary cortical neurons cultured over 4 weeks on the transparent PEDOT:PSS electrodes. This work shows that microelectrodes composed of PEDOT:PSS are very promising as a new tool for both electrophysiology and fluorescence microscopy studies on neuronal cell cultures.
Abstract: In this study, we present the microfabrication and characterization of a transparent microelectrode array (MEA) system based on PEDOT:PSS for electrophysiology. The influence of the PEDOT:PSS electrode dimensions on the impedance was investigated and the stability over time under physiological environment was demonstrated. A very good transparency value was obtained by our system displaying one of the best impedance and transmittance values when compared to other transparent MEA. After biocompatibility validation, we successfully recorded spontaneous neuronal activity of primary cortical neurons cultured over 4 weeks on the transparent PEDOT:PSS electrodes. This work shows that microelectrodes composed of PEDOT:PSS are very promising as a new tool for both electrophysiology and fluorescence microscopy studies on neuronal cell cultures.
Susloparova A., Halliez S., Begard S., Colin M., Buée L., Pecqueur S., Alibart F., Thomy V., Arscott S., Pallecchi E., Coffinier Y.*
© 2019-2025 Sébastien Pecqueur