
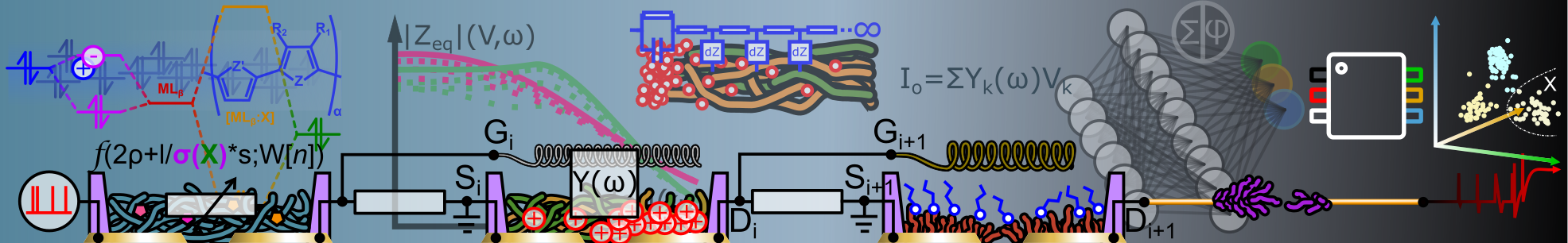





 Abstract: Microelectrode Arrays (MEAs) are popular tools for in vitro extracellular recording. They are often optimized by surface engineering to improve affinity with neurons and guarantee higher recording quality and stability. Recently, PEDOT:PSS has been used to coat microelectrodes due to its good biocompatibility and low impedance, which enhances neural coupling. Herein, we investigate on electro-co-polymerization of EDOT with its triglymated derivative to control valence between monomer units and hydrophil ic functions on a conducting polymer. Molecular packing, cation complexation, dopant stoichiometry are governed by the glycolation degree of the electro-active coating of the microelectrodes. Optimal monomer ratio allows fine-tuning the material hydrophi licity and biocompatibility without compromising the electrochemical impedance of microelectrodes nor their stability while interfaced with a neural cell culture. After incubation, sensing readout on the modified electrodes shows higher performances with respect to unmodified electropolymerized PEDOT, with higher signal-to-noise ratio (SNR) and higher spike counts on the same neural culture. Reported SNR values are superior to that of state-of-theart PEDOT microelectrodes and close to that of state-of-t he-art 3D microelectrodes, with a reduced fabrication complexity. Thanks to this versatile technique and its impact on the surface chemistry of the microelectrode, we show that electro-co-polymerization trades with manycompound properties to easily gathe r them into single macromolecular structures. Applied on sensor arrays, it holds great potential for the customization of neurosensors to adapt to environmental boundaries and to optimize extracted sensing features.
Abstract: Microelectrode Arrays (MEAs) are popular tools for in vitro extracellular recording. They are often optimized by surface engineering to improve affinity with neurons and guarantee higher recording quality and stability. Recently, PEDOT:PSS has been used to coat microelectrodes due to its good biocompatibility and low impedance, which enhances neural coupling. Herein, we investigate on electro-co-polymerization of EDOT with its triglymated derivative to control valence between monomer units and hydrophil ic functions on a conducting polymer. Molecular packing, cation complexation, dopant stoichiometry are governed by the glycolation degree of the electro-active coating of the microelectrodes. Optimal monomer ratio allows fine-tuning the material hydrophi licity and biocompatibility without compromising the electrochemical impedance of microelectrodes nor their stability while interfaced with a neural cell culture. After incubation, sensing readout on the modified electrodes shows higher performances with respect to unmodified electropolymerized PEDOT, with higher signal-to-noise ratio (SNR) and higher spike counts on the same neural culture. Reported SNR values are superior to that of state-of-theart PEDOT microelectrodes and close to that of state-of-t he-art 3D microelectrodes, with a reduced fabrication complexity. Thanks to this versatile technique and its impact on the surface chemistry of the microelectrode, we show that electro-co-polymerization trades with manycompound properties to easily gathe r them into single macromolecular structures. Applied on sensor arrays, it holds great potential for the customization of neurosensors to adapt to environmental boundaries and to optimize extracted sensing features.
Ghazal M., Susloparova A., Lefebvre C., Daher Mansour M., Ghodhbane N., Melot A., Scholaert C., Guérin D., Janel S., Barois N., Colin M., Buée L., Yger P., Halliez S., Coffinier Y.*, Pecqueur S.*, Alibart F.


 Abstract: Recently, the development of electronic devices to extracellularly record the simultaneous electrical activities of numerous neurons has been blooming, opening new possibilities to interface and decode neuronal activity. In this work, we tested how the u se of EDOT electropolymerization to tune post-fabrication materials could optimize the cell/electrode interface of such devices. Our results showed an improved signal-to-noise ratio, better biocompatibility, and a higher number of neurons detected in com parison with gold electrodes. Then, using such enhanced recordings with 2D neuronal cultures combined with fluorescent optical imaging, we checked the extent to which the positions of the recorded neurons could be estimated solely via their extracellular signatures. Our results showed that assuming neurons behave as monopoles, positions could be estimated with a precision of approximately tens of micrometers.
Abstract: Recently, the development of electronic devices to extracellularly record the simultaneous electrical activities of numerous neurons has been blooming, opening new possibilities to interface and decode neuronal activity. In this work, we tested how the u se of EDOT electropolymerization to tune post-fabrication materials could optimize the cell/electrode interface of such devices. Our results showed an improved signal-to-noise ratio, better biocompatibility, and a higher number of neurons detected in com parison with gold electrodes. Then, using such enhanced recordings with 2D neuronal cultures combined with fluorescent optical imaging, we checked the extent to which the positions of the recorded neurons could be estimated solely via their extracellular signatures. Our results showed that assuming neurons behave as monopoles, positions could be estimated with a precision of approximately tens of micrometers.
Ghazal M., Scholaert C., Dumortier C., Lefebvre C., Barois N., Janel S., Çağatay Tarhan M., Colin M., Buée L., Halliez S., Pecqueur S., Coffinier Y., Alibart F.*, Yger P.*

Abstract: The development of electronic devices such as microelectrode arrays (MEAs), used to record extracellularly simultaneous electrical activity of large populations of neurons is blooming. To enhance the quality of the recordings, the use of electrode made o f conducting polymer such as PEDOT has recently emerged for optimizing the performance of microelectrodes due to its mixed ionic electronic conduction, biocompatibility and low impedance. However, the extent to which these new interfaces can help the alg orithmic pipelines of spike sorting, i.e. turning extracellular potentials into individual spike trains remains unexplored. To address this issue, we checked if the physical positions of the neurons could be reliably inferred from extracellular electrica l recordings obtained by MEAs, and thus be exploited by downstreams spike sorting algorithms. To do so, we combine high resolution images of neuronal tissues and dense recordings performed via high performant electropolymerized electrodes based MEAs. Fir stly, we report the use of EDOT electropolymerization to tune post-fabrication material and geometrical parameters of passive microelectrodes. The process optimizes the cell/electrode interface by decreasing its impedance and improving its affinity with neurons: results demonstrate a better biocompatibility and improved signal-to-noise ratio (SNR) (up to 40 dB). Thanks to the higher SNR, we were able to detect more cells in comparison with gold electrodes from the same neural network by using spike sort ing. Hence, the higher number of cells detected will lead into more accurate analysis of the localization of the active neurons on top of MEA. Secondly, by using these high performant MEAs, we investigated the possibility to accurately estimate the posit ions of the neurons solely from extracellular recordings by studying the correlation between electrical activity (obtained via spike sorting), optical imaging (Fluorescent) and Scanning Electron Microscopy (SEM) of neural networks cultured on MEAs. By us ing the SpykingCircus software to spike sort the extracellular recordings, we estimated the positions of the neurons either by using the center of mass of their electrical signatures, or by inferring the positions assuming cells would behave as monopoles . By superposition of the fluorescent and the SEM images, we compared the observed physical positions of the neurons with the ones predicted by the two aforementioned methods. This approach showed the high accuracy of the monopole hypothesis compared to the center of mass. In this work, we showed how the use of a material engineering technique for optimizing state of art MEAs can enhance the quality of the recordings. Hence, the correlation of these high quality recordings with optical imaging paves the way towards new algorithmic possibilities for spike sorting algorithms that could make use of a more reliable estimation of neuronal positions.
Ghazal M., Scholaert C., Lefebvre C., Barois N., Janel S., Çağatay Tarhan M., Colin M., Buée L., Halliez S., Pecqueur S., Coffinier Y., Alibart F., Yger P.

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract: Most of today's strategies to interface biology with electronic hardware are based on layered architectures where the front-end of sensing is optimized separately from the back-end for processing/computing signals. Alternatively, biological systems are c apitalizing on distributed architecture where both sensing and computing are mix together and co-optimized. In this talk, we will present our strategy to implement bio-sensing of electroactive cells in a neuromorphic perspective. We will present how orga nic electrochemical transistors can be used to record electrical signals from neural cells. We will show various strategies capitalizing on the versatility of organic materials synthesis and organic device fabrication to tune and adapt the functionalitie s of such bio-sensors. We will then present how these strategies can be efficiently used to realize computing functions directly at the interface with biology. Notably, we will illustrate how a network of ionic sensors can implement the reservoir computi ng concept, a powerful neuromorphic computing approach of particular interest for dynamical signal processing.
Alibart F., Ghazal M., Janzakova K., Kumar A., Susloparova A., Halliez S., Colin M., Buée L., Guérin D., Dargent T., Coffinier Y., Pecqueur S.

Abstract: The recent progress in the extracellular microelectrode arrays (MEAs) have greatly improved our ability to probe cellular electrophysiological activities. Nevertheless, passive MEAs are subject to small signal-to-noise ratio and small potential detection . Recently, organic electrochemical transistors (OECTs) have been identified as a promising device architecture to improve extracellular potentials recording in electroactive cells culture both in-vitro and in-vivo. In addition to unique properties of in terest for electrophysiology such as biocompatibility, transparency and flexibility, OECTs operating principle is based on the transduction of ionic currents in the biological medium into electronic currents in the organic semiconductor (e.g. PEDOT:PSS) via electrochemical coupling. The transconductance represents an important figure of merit of OECTs and depends on geometrical and material parameters that rules largely OECTs performances for sensing electrophysiological signals. However, as an organic electronic technology, larger device-property distributions are often encountered with respect to the one of metal- or inorganic-based technologies, inherent to the very nature of the soft organic materials involved in the OECTs transduction process. Her e, we explore the possibility to tune post-fabrication material and geometrical parameters of OECTs with electropolymerization of EDOT. We show that this strategy can be used to simultaneously improve OECT transconductance and its geometrical capacitance . The addressed OECT chips were micro-fabricated on a glass substrates with spin coated PEDOT:PSS. Electropolymerization of EDOT on top of spin-coated PEDOT:PSS was carried on with both fix voltage and ramp voltage techniques. A detail impedance analysis was performed during OECTs functionalization. DC electrical characterizations was used to correlate the transconductance and capacitance tuning due to electropolymerization and to assess device performances improvements. Scanning Electron Microscopy (SE M) was used to correlate morphological changes due to electropolymerization with the enhancement in the transconductance and capacitance of the OECTs. Finally, we performed bio-compatibility assessment between primary neural cells culture and the differe nt possible monomers used for electropolymerization to evaluate the possibility to improve affinity between cultured neurons and electropolymerized materials. The key novelty of this material engineering technique is to propose a promising method for tun able OECTs sensors development. For instance, this back-end-of-line tuning technique can reduce chip variability in terms of performance yield and bring OECTs technology to the next maturity level. Furthermore, such flexibility can enable matching the el ectrochemical impedance of the device to the one of the cells, and in the future promote exploratory sensing missions, merging brain-inspired information processing with neuro-sensing.},
Ghazal M., Susloparova A., Halliez S., Colin M., Buée L., Coffinier Y., Pecqueur S., Dargent T., Alibart F.


 Abstract: In this study, we present the microfabrication and characterization of a transparent microelectrode array (MEA) system based on PEDOT:PSS for electrophysiology. The influence of the PEDOT:PSS electrode dimensions on the impedance was investigated and the stability over time under physiological environment was demonstrated. A very good transparency value was obtained by our system displaying one of the best impedance and transmittance values when compared to other transparent MEA. After biocompatibility validation, we successfully recorded spontaneous neuronal activity of primary cortical neurons cultured over 4 weeks on the transparent PEDOT:PSS electrodes. This work shows that microelectrodes composed of PEDOT:PSS are very promising as a new tool for both electrophysiology and fluorescence microscopy studies on neuronal cell cultures.
Abstract: In this study, we present the microfabrication and characterization of a transparent microelectrode array (MEA) system based on PEDOT:PSS for electrophysiology. The influence of the PEDOT:PSS electrode dimensions on the impedance was investigated and the stability over time under physiological environment was demonstrated. A very good transparency value was obtained by our system displaying one of the best impedance and transmittance values when compared to other transparent MEA. After biocompatibility validation, we successfully recorded spontaneous neuronal activity of primary cortical neurons cultured over 4 weeks on the transparent PEDOT:PSS electrodes. This work shows that microelectrodes composed of PEDOT:PSS are very promising as a new tool for both electrophysiology and fluorescence microscopy studies on neuronal cell cultures.
Susloparova A., Halliez S., Begard S., Colin M., Buée L., Pecqueur S., Alibart F., Thomy V., Arscott S., Pallecchi E., Coffinier Y.*
© 2019-2025 Sébastien Pecqueur